Featured Experts

Ben Wiley
Duke University Professor of Chemistry

Ben Wiley
Duke University Professor of Chemistry
Ben Wiley is a Professor of Chemistry at Duke University where he studies materials and methods for improving the efficiency of water electrolysis to create hydrogen for energy use.
Featured In:
In this Episode
Hydrogen is uniquely qualified as a storage of clean energy because it is abundant – the most abundant element in the universe – and it can be produced using renewable energy.
When consumed in a fuel cell, its only byproduct is water, making it ideal for our net-zero emissions future.
Hydrogen, however, also comes with unique challenges, such as high inefficiency during production and transportation difficulties.
Climate Now host Dr. Ozak Esu details the benefits and trade-offs of hydrogen with the help of Duke University Professor of Chemistry Dr. Ben Wiley.
Related Media:
Climate Now: Jul 27, 2021
Hydrogen Electrolysis with Ben Wiley
Declining renewable energy costs have sparked a renewed interest in green hydrogen, which has the potential to decarbonize sectors in which electricity cannot. Because hydrogen doesn’t occur by itself on Earth, it must be separated from other elements, s


Climate Now: Jun 25, 2021
Net-Zero by 2050 with Eric Larson
What are the possible paths and necessary steps to achieve net-zero emissions in the United States by 2050? Which energy sources could sufficiently decrease our reliance on natural gas and oil to meet that target? And how much will those new energy sources nee


Research Ep 1
Net-Zero by 2050
Pledges to achieve “net-zero” emissions are proliferating from companies and countries alike. However sincere these commitments may be, they rarely include specific plans to achieve that ambition. The Net-Zero America Report from Princeton Universi


Climate Now: Jan 25, 2022
Re-imagining Heavy-Duty Trucking with Hydrogen and Carbon Capture
Heavy-duty, long-haul trucks – known as Class 8 trucks – account for more than 1 billion tons of carbon dioxide emissions worldwide each year. Electrification, while a practical option for most of the trucking industry (see last week’s episo
Episode Transcript
Hydrogen fuel, what is it? How is it used, and how can it help us move towards a zero-carbon future? In another episode (Net-Zero by 2050), we discussed potential pathways to reaching a net-zero America by 2050. Hydrogen is mentioned in this report as a means to store energy without relying heavily on batteries. But what does that mean? In this episode, we are going to provide a deeper insight into hydrogen, how it is made, how it is used, some of the latest advancements, along with current constraints to this technology.
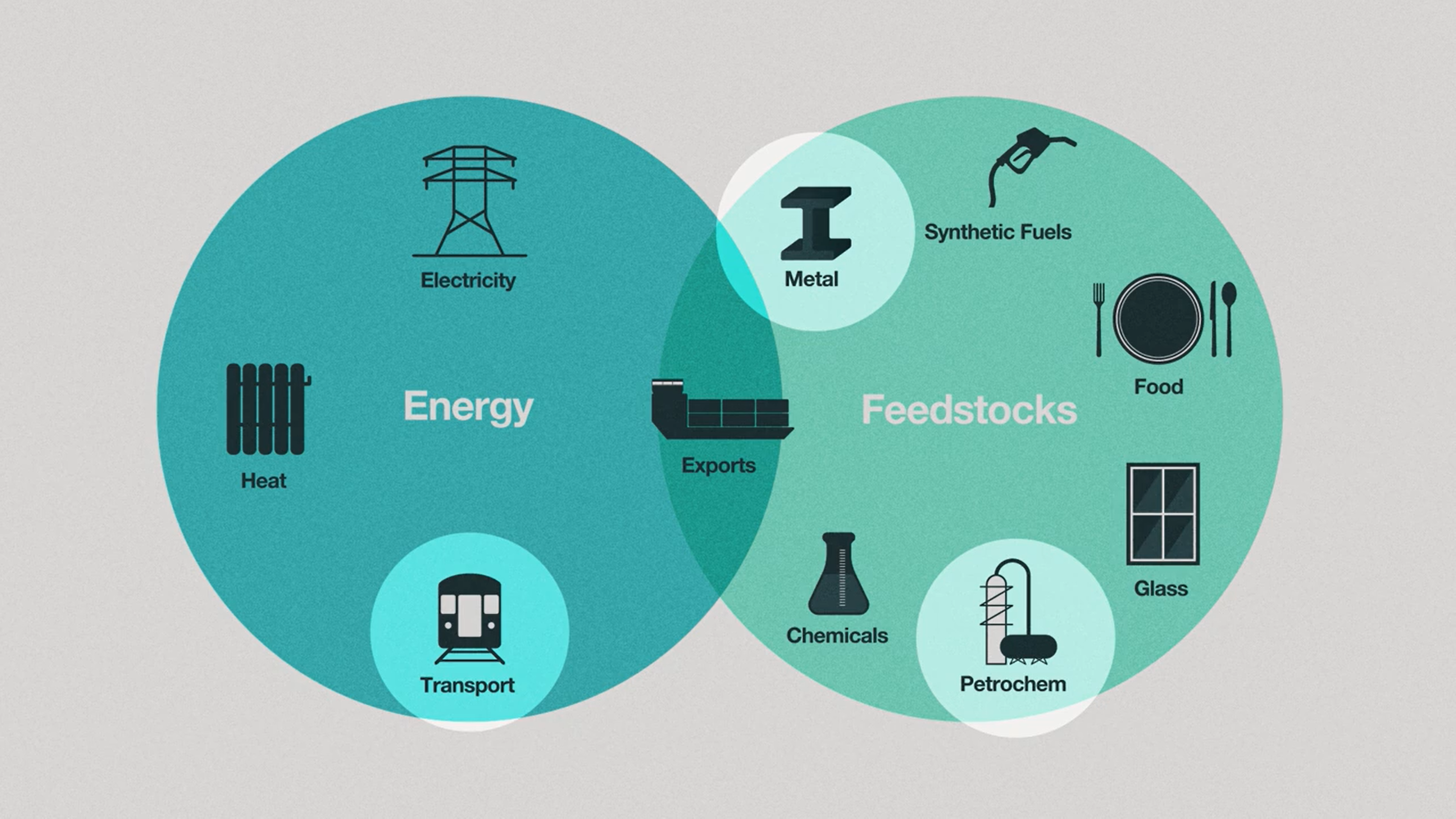
So what’s so special about hydrogen? Well, hydrogen can be used not only as energy, but also as feedstock and a means to store energy. In this diagram [1,7], we see that hydrogen can be used to power vehicles, create fertilizer, and process metals like iron and steel. Another added benefit of hydrogen technology is that it can, in theory, be used to power sectors of the economy that are harder to electrify, such as long-haul shipping[2], aviation[3], and various industrial processes[4].
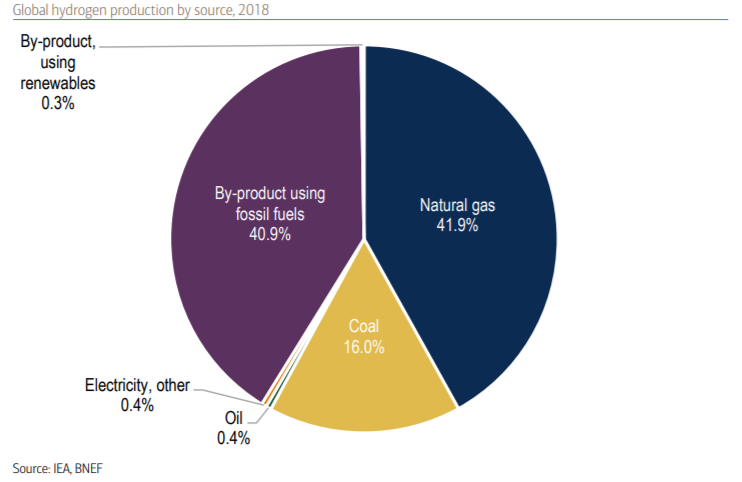
But why hydrogen? Hydrogen is the most abundant element in the universe. However, on earth, it is not naturally found all by itself. It is usually attached to other elements, such as oxygen, which creates water, or carbon to create hydrocarbons like methane. Because hydrogen does not exist alone in nature, it needs to be extracted. This pie chart produced by data from the International Energy Agency [4,7] tells us that currently 99% of the hydrogen we extract is a byproduct of fossil fuels, natural gas and coal.
HYDROGEN PRODUCTION
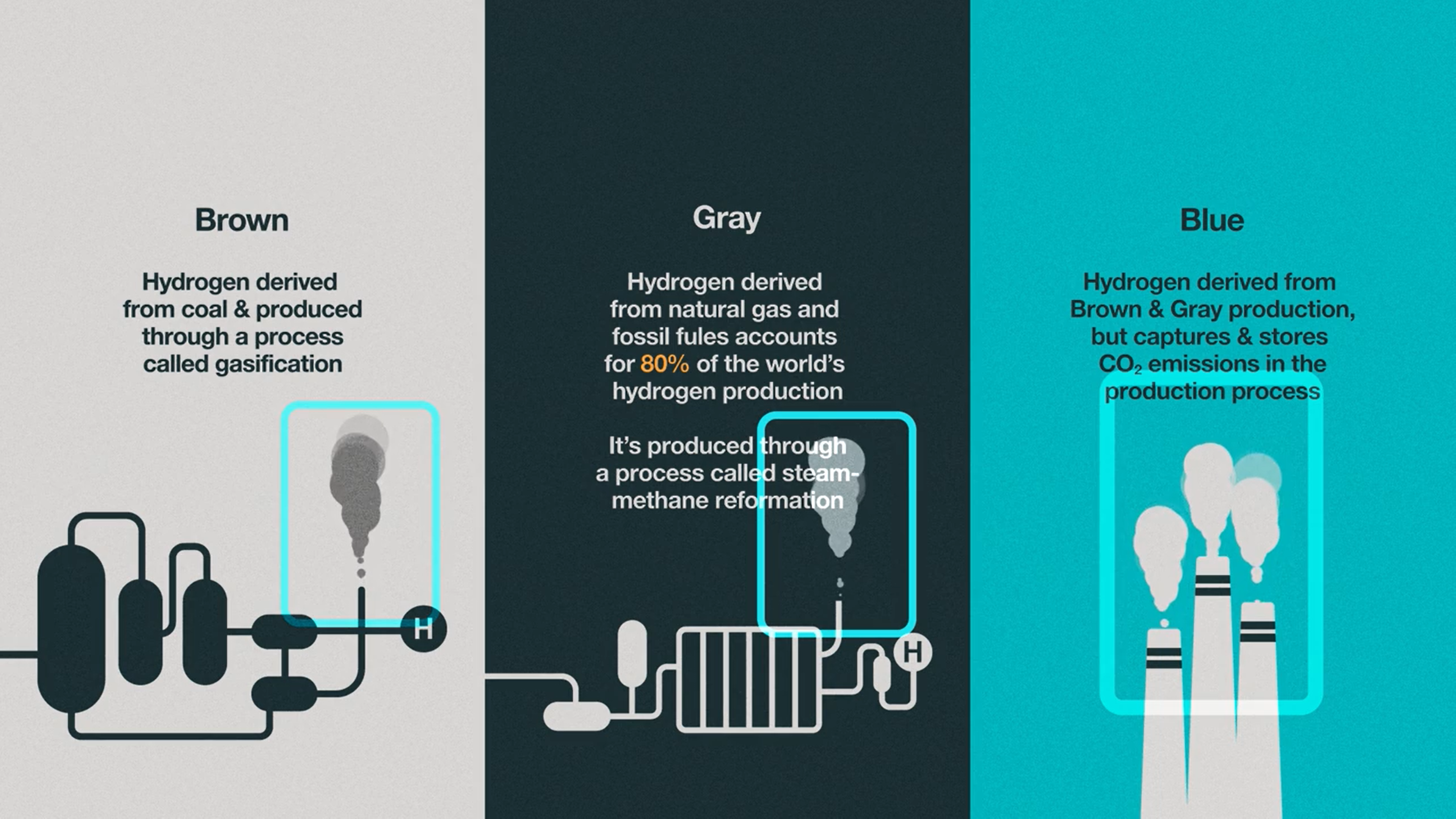
To distinguish each source of hydrogen production scientists use a color classification system: brown, gray, blue, and green. Hydrogen derived from coal is known as brown hydrogen and is produced via a process known as gasification [5]. Gray hydrogen, which accounts for about 80% of the world’s hydrogen production, is produced via steam methane reformation and is derived from natural gas and other fossil fuels [5]. Both brown and gray hydrogen release CO2 as byproducts, thus contributing further to climate change impacts. However, blue hydrogen involves the capture and storage of this CO2 byproduct [5]. But if the aim is to remove harmful greenhouse gases from the atmosphere, clean hydrogen fuel cannot be created through fossil fuel burning.
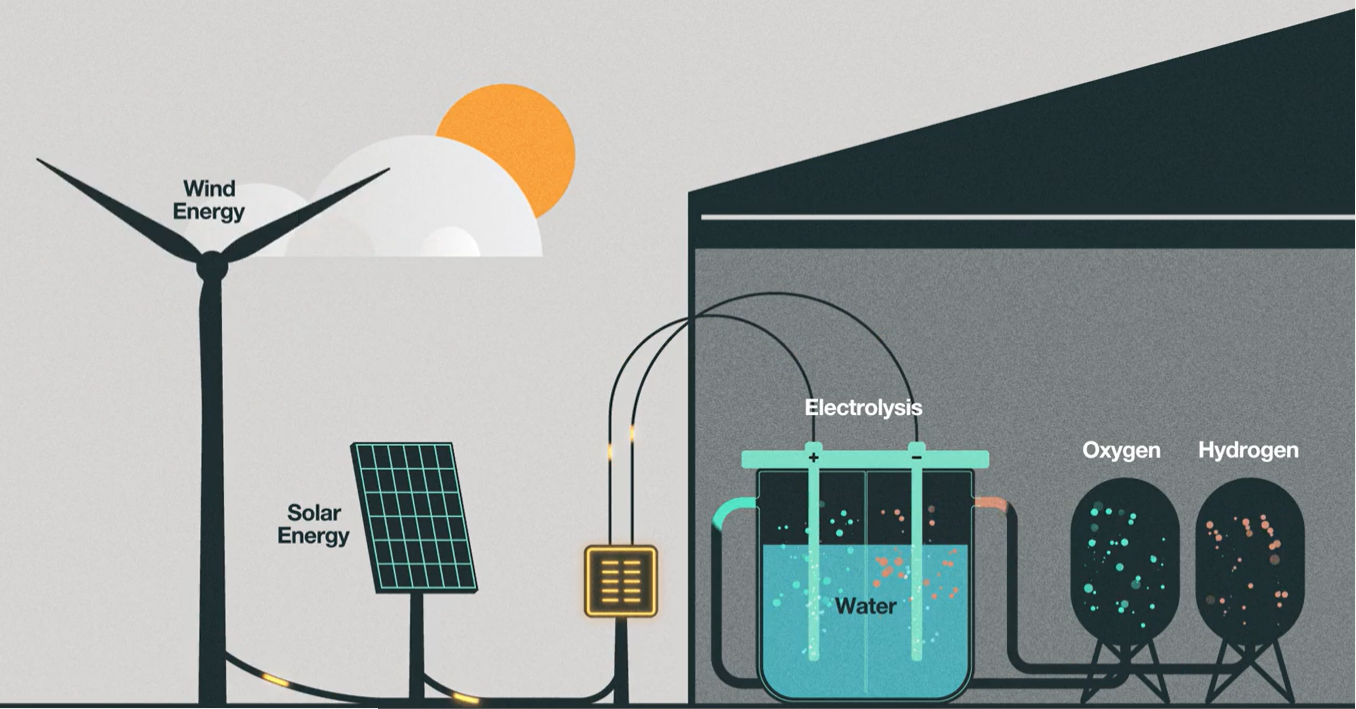
In the effort to eliminate fossil fuels, green hydrogen takes all of this a step further via a process known as electrolysis, also known as water-splitting. Here an electrolyzer splits freshwater particles into hydrogen and oxygen using an electric current, if renewable energy like wind or solar is used, as the electricity source, then the derived hydrogen is known as green hydrogen [5]. Another form of green hydrogen involves biomass gasification. This involves growing high energy plants and then converting them at more than 700 degrees Celsius temperatures to hydrogen, carbon monoxide and carbon dioxide [6]. Although this method does produce some carbon dioxide, because the plant absorbs carbon dioxide while it’s being grown, it results in lower carbon emissions in comparison to brown and gray hydrogen.
HYDROGEN FUEL CELLS
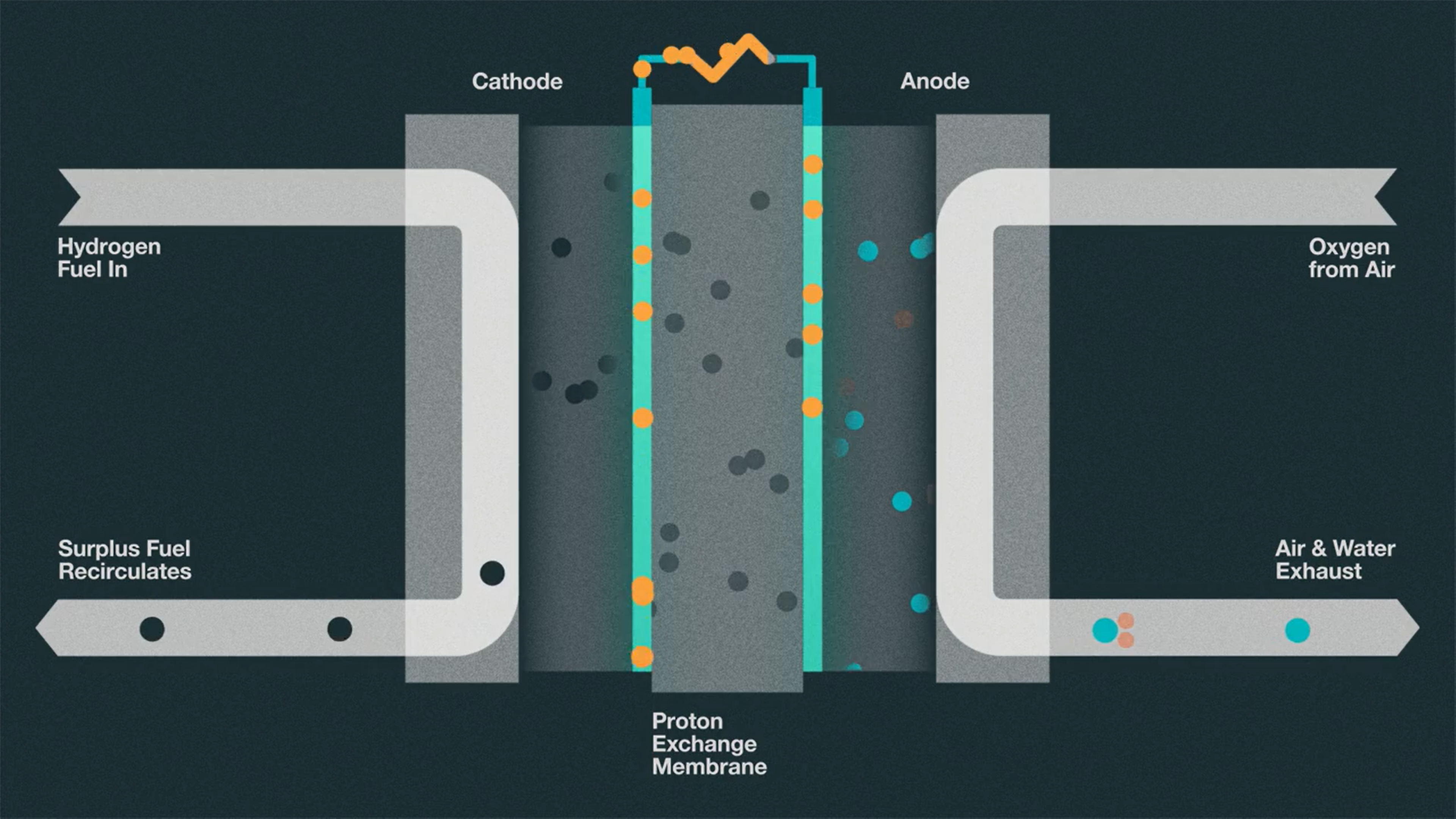
So now that we know the various means by which hydrogen is produced, let’s talk about how hydrogen can be converted into electricity using fuel cells, and the pros and the cons of those processes. Due to the fact that hydrogen itself can only store energy, it must be processed through a fuel cell in order to be used for power. At the core of this technology, we see that hydrogen and oxygen atoms are bonded to produce energy and water as a byproduct [7]. When we take a look inside a fuel cell, we see that we have an input of hydrogen on the cathode side and an input of oxygen on the anode side [8]. A catalyst is used to split hydrogen into its electrons and protons. The cathode is what holds onto and attracts the hydrogen electrons, while the protons pass through a membrane as they are attracted to the anode where the oxygen is. Then, the electrons are pushed through a circuit to produce an electric current and heat. The hydrogen electrons are then reunited with the protons and with oxygen to form water. Cells like this can then be stacked and used as a source of energy inside vehicles or as power for energy plants.
Hydrogen Fuel Cell:
STORAGE
But what do we do with hydrogen when we don’t need it quite yet? The most expensive option is to store it in pressurized tanks. It can even be liquified and stored in cryogenic tanks [1]. But what you probably didn’t know is that we can also take hydrogen and store it underground in salt caverns [1]. This method has been proven to be safe and reliable considering it doesn’t require high pressurization, and it’s also the cheapest way to store hydrogen for long periods of time.
LIMITATIONS
However, using hydrogen as a fuel source comes with several disadvantages.
- Fresh water is a limited resource. Can we afford to use our fresh water resources for electrolysis to produce hydrogen?[7]
- The process from extracting hydrogen, transporting it and consuming it is highly inefficient. Between extraction and consumption around 70% of energy is lost. The main reason why hydrogen remains a relatively feasible alternative to fossil fuels is because it is energy dense. This means that it can store a lot of energy in a small volume [5].
- Additionally, hydrogen is a highly flammable and odorless gas. Should a leak go undetected in a potentially explosive environment, the results could be disastrous [5].
- When it comes to biomass gasification, this process still results in greenhouse gases, like carbon dioxide and carbon monoxide [6].
- We also do not currently have enough infrastructure such as fueling stations or pipelines to distribute hydrogen across the country [4].
- And lastly, it’s simply still not economical at this time. Using hydrogen only makes sense at scale and it stills needs more financial support to get there [4].
COSTS
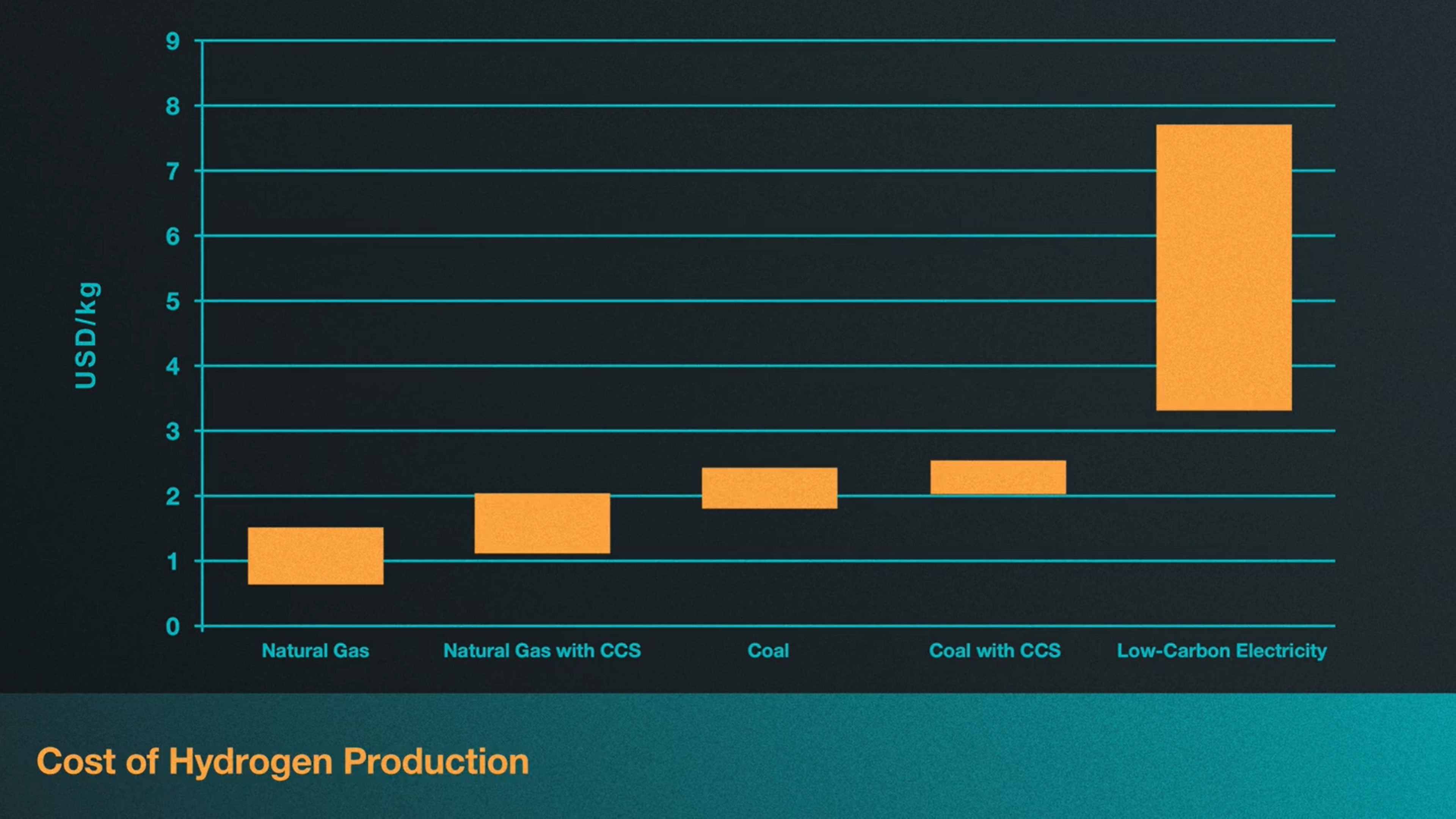
What makes things even more complicated is that the cost of replacing fossil fuels for hydrogen is highly dependent on who’s producing it and where. Regional variations in hydrogen production costs depend on factors such as prices for fossil fuels, electricity and carbon. In fact, according to a report released by the International Energy Agency in 2019, natural gas without carbon capture and storage is currently the most economic option for hydrogen production in most parts of the world, with costs being as low 1 USD per kilogram of hydrogen in the Middle East. In this chart, we see that the most expensive option globally is green hydrogen, ranging from 3-7.5 USD per kilogram[4]. However, green hydrogen may be more attractive in regions that already rely heavily on renewable resources or nuclear power plants, especially if your current input costs of natural gas are relatively high. Though this isn’t to say that investments aren’t being made to lower the costs of hydrogen fuel.
ADVANCEMENTS
Governments and universities around the world are investing more and more into making hydrogen as efficient as possible. In May, 2020 Ben Wiley, a professor of chemistry at Duke University, and his colleagues, published a study that demonstrates the potential of using nickel microwire mesh as a more productive electrode in the water splitting process [9]. A more productive electrode means lower overall costs of electrolysis. Let’s hear from Ben on how they were able to make this possible.
Ben Wiley:
If you try to run this thing really hard, pretty soon your anode or cathode, one or the other, is going to be covered with gas and you won’t be able to pass any more electricity through it. And so we thought, well, how about you run a flow of the electrode, like fluid flow to drive the gas off, and then you can imagine, okay, a metal plate is, is good, but what if I make the surface area of that metal plate, you know, way bigger, by splitting it into tiny nanowires? And so you can imagine taking like a piece of steel wool, that’s gonna have a lot more surface area than a piece of steel, right? And that means if it has more surface area, there’s more surface area to pass electricity to the oxygen or hydrogen atoms in the water.
We looked at three different length scales of these kinds of porous materials made of nickel. In this case, since nickel is pretty good for water splitting, we use like a nickel foam, a nickel microwire mesh and a nickel nanowire mash or felt. And we thought the nanowire felt would be the best because it has the highest surface area, but it wasn’t, it was very tight and trapped the bubbles from water splitting. So it was the microwire mesh that we could flow the bubbles out. So that ended up being the best electrode.
As it stands today, the cheapest way to make industrial quantities of hydrogen is through steam methane reformulation where methane is broken down using very hot steam [10]. Based on Wiley’s results, by improving the materials used to make electrolyzers, water spitting can become a viable alternative to producing massive amounts of hydrogen, thanks to higher production rates, meaning lower costs [10]. But what about lithium ion batteries? Why even bother with hydrogen when we can use batteries to electrify our vehicles and store energy? Well, the answers are fairly simple.
Ben Wiley:
For long durations of storage it makes a lot more sense to store the energy in hydrogen because you can just store it in a big steel tank rather than in a gigantic battery. And then you can run your fuel cell off that stored hydrogen for a very long time. Whereas batteries they’ll typically discharge them in a matter of hours.
Another added benefit is that vehicles powered by hydrogen are capable of being refueled at much faster rates than electric cars [11]. An average electric car can take between 45 minutes to several hours to fully refuel. Hydrogen cars on the other hand can be refueled within five minutes [5]. What’s even better is that you can go longer distances on hydrogen fuel, it’s lightweight and it takes up considerably less space than a battery would. This makes hydrogen particularly attractive for long haul shipping and aviation. In fact, the larger the vehicle, the more sense hydrogen makes cost and energy use-wise.
According to this map pulled from the 2020 report by the World Energy Council, Germany [12], 42 countries are currently moving in a direction to create national strategies to support green hydrogen production, while 9 countries are already there. By 2025, it is expected that these strategies will likely cover countries representing over 80% of global GDP. So, from a policy perspective, green hydrogen is well on its way to play a significant role in electrifying our future and going net-zero by 2050. Moving forward, it will be important to keep an eye on how advances in technology continue to lower cost for this energy sector. How policy supports this transition and how fast infrastructure can be built or converted to support hydrogen expansion [13].
Check out our full-length podcast with Ben Wiley, where we go into further detail about the current state of hydrogen technology, or check out our newsletter. To sign up for new releases and more visit climatenow.com. Thanks and see you next time.
Sources
[1] 00:51 and 5:21 Bruce S, Temminghoff M, Hayward J, Schmidt E, Munnings C, Palfreyman D, Hartley P (2018) National Hydrogen Roadmap. CSIRO, Australia.
[2] 1:10 Navistar. (2021, January 27). Navistar Collaborates with General Motors and OneH2 to Launch Hydrogen Truck Ecosystem [Press Release]. https://www.internationaltrucks.com/-/media/Project/International-Trucks/International-Trucks/USA/Alternate-Fuel/Hydrogen-Fuel-Cell/HydrogenNR-1-25-21FINAL.pdf
[3] 1:11 ZeroAvia. (2020, September 25). ZeroAvia Completes World First Hydrogen-Electric Passenger Plane Flight [Press Release]. https://www.zeroavia.com/press-release-25-09-2020
[4] 1:13 and 1:41 and 6:54, 7:34 IEA (2019), The Future of Hydrogen, IEA, Paris https://www.iea.org/reports/the-future-of-hydrogen
[5] 2:08 and 6:24, 11:34CNBC (Director). (2020, December 03). What is green hydrogen and will it power the future? [Video file]. https://www.youtube.com/watch?v=aYBGSfzaa4c
[6] 3:35 and 6:46 Hydrogen production: Biomass gasification. (n.d.). Energy.gov. https://www.energy.gov/eere/fuelcells/hydrogen-production-biomass-gasification
[7] 4:20 and 5:59 BofA Global Investing. (2020, September 24) The Special 1 – Hydrogen Primer. https://www.bofaml.com/content/dam/boamlimages/documents/articles/ID20_1005/hydrogen_final.pdf
[8] 4:31 Let’s Grow Up (2020, October 15). How does a hydrogen fuel cell work? | what is hydrogen fuel cell | hydrogen cell explain [Video file]. https://www.youtube.com/watch?v=a4pXAmljdUA
[9] 8:43 Yang, F., Kim, M. J., Brown, M., Wiley, B. J. (2020). Alkaline Water Electrolysis at 25 A cm−2 with a Microfibrous Flow‐through Electrode. Advanced Energy Materials. https://doi.org/10.1002/aenm.202001174
[10] 10:27 Duke University. (2020, May 26). Flow-through electrodes make Hydrogen 50 times faster. Eurekalert.org. https://www.eurekalert.org/pub_releases/2020-05/du-fem052620.php
[11] 11:23 Bmw. (2020, September 22). Hydrogen cars, fuel cells, etc.: What you need to know. https://www.bmw.com/en/innovation/how-hydrogen-fuel-cell-cars-work.html
[12] 12:03 Albrecht, U., Bünger, U., Michalski, J., Raksha, T., Wurster, R., & Zerhusen, J. (2020, September) International Hydrogen Strategies – weltenergierat. https://www.weltenergierat.de/wp-content/uploads/2020/10/WEC_H2_Strategies_finalreport.pdf
[13] 12:53 Hydrogen Council. (2020, January 20). Path to hydrogen competitiveness: A cost perspective. https://hydrogencouncil.com/wp-content/uploads/2020/01/Path-to-Hydrogen-Competitiveness_Full-Study-1.pdf Copenhagen Centre on Energy Efficiency.